Leading the Way in Redefining Wnt-Driven Cancer Therapy
Iterion Therapeutics is a clinical-stage, biopharmaceutical company dedicated to revolutionizing healthcare through novel drug discovery and innovation.
Tegavivint:
Drugging the Undruggable
Tegavivint targets TBL1, a necessary co-activator for Wnt-activated gene transcription, thereby blocking beta-catenin’s oncogenic activity while avoiding the toxicities that have limited upstream Wnt inhibitors. This unique mechanism positions Tegavivint as the most advanced Wnt/beta-catenin inhibitor in development, with broad applicability across more than 500,000 cancers annually.
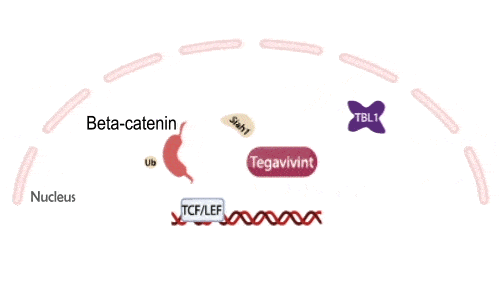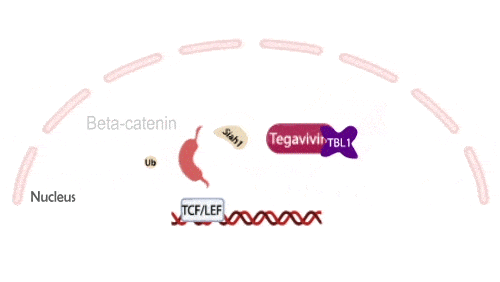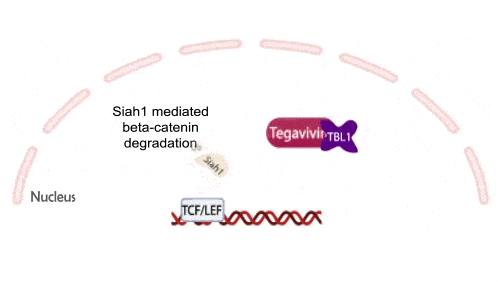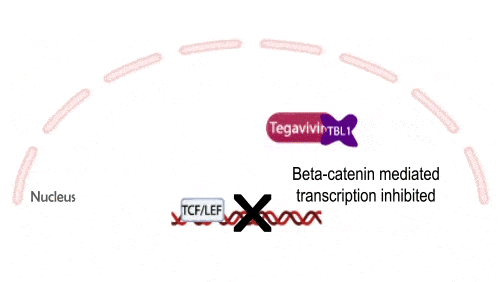
Tegavivint in the Clinic
Iterion’s primary clinical focus is advanced hepatocellular carcinoma (HCC), where approximately 40% of patients harbor Wnt/beta-catenin mutations and no targeted therapies exist. In a Phase 1 study, Tegavivint achieved partial responses and durable disease control in heavily pre-treated, Wnt-mutated HCC patients, supported by ctDNA reductions and improvements in liver function. With strong safety, excellent tolerability, and outcomes superior to late-line TKIs, Tegavivint is positioned to become the first targeted therapy for Wnt-mutated HCC. Beyond HCC, Iterion is advancing expansion opportunities into other Wnt-driven tumors including CRC, representing a multi-billion-dollar market.
The company also has deep collaborative relationships with leading institutions studying tegavivint in complementary investigator-sponsored trials.

